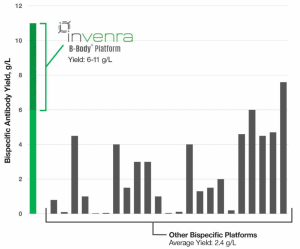
Unrivaled Bispecific Yield
The B-Body Platform overcomes the challenges in discovery and manufacturing of bispecific antibodies with some of the highest antibody yields in both transient and stable cell line expression. Evaluation of a range of production methods from engineered CHO pools in shake flasks to fed-batch production has yielded 6-11 grams per liter, substantially higher than any other known bispecific platform. Yields of up to 1.8g/L/day or higher are possible in perfusion cell culture. In addition to high yield, the B-Body Platform also solves for:
- Validated Production – Record yields achieved with two independent, commercially produced stable cell lines including ATUM Leap-In Transposase® and miCHO® Stable Cell Line Development
- Proper Assembly – Correct heterodimerization of heavy:heavy and heavy:light chains for maximum Ab yield and simple CMC
- 95% Single-Pass Purity for Discovery – A unique CH1 affinity handle enables simple purification for rapid discovery
- 100% Purity After CEX Purification – Samples assayed by HPLC indicated high yield and homogeneity following a two column protocol
- Compatibility with Conventional CMC – Human IgG-like scaffold, fully compatible with conventional Protein A, IEX, UF, etc.
- Therapeutic-Ready – With high solubility, thermal stability, minimal side product and low viscosity. B-Body Bispecifics are production-ready
Compatible with Sub-Q Delivery
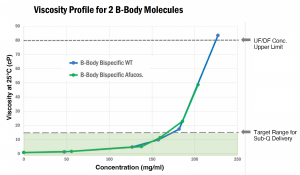
The high solubility, excellent stability, and low viscosity of B-Body Bispecifics make them ideally suited for subcutaneous delivery consideration. In a comparison of two B-Body Bispecifics (with high and low fucose), antibody concentrations required for Sub-Q were achieved with similar viscosity values well below ultrafiltration/diafilitration limits.
- Sub-Q Compatible Viscosity – Up to 170mg/ml or higher without exceeding viscosity limits
- Within Range of UF/DF Production – Sub-Q concentrations possible without exceeding UF/DF limits
- Excellent Solubility – Higher concentrations (>200mg/ml) can be achieved if required for specific applications
B-Body: Innovative By Design
CMC Driven Innovations:
![]()
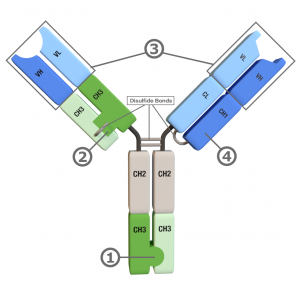
Fc Region: Clinically Validated Knobs-into-Holes
- Drives heavy chain heterodimerization
![]()
Fab Arm: Proprietary CH3 Domains to Drive Heterodimerization
- Substitutes for CH1/CL in one Fab Arm
- Solves light chain mispairing issue
- Novel CH3 mutations, without knobs-into-holes or charge pairs
- pI asymmetry streamlines polishing steps
Antibody Discovery and CMC Innovations:
![]()
Heavy & Light Chains: Proprietary Symmetrical Inversions
- Enhances expression yield and stability
- Facilitates one-step purification
- Compatible with a wide variety of variable domain sources
![]()
Sole CH1 Domain Allows Another Level of Purification Beyond Protein A/IEX
- Facilitates one-step purification with anti-CH1 resins
- Simplifies in-format discovery
- Removes knob-knob homodimers and other impurities in one pass